Ideal Tips About How To Draw A Dipole Moment
The dipole moment of a molecule can be calculated by another primary equation that is mentioned below:
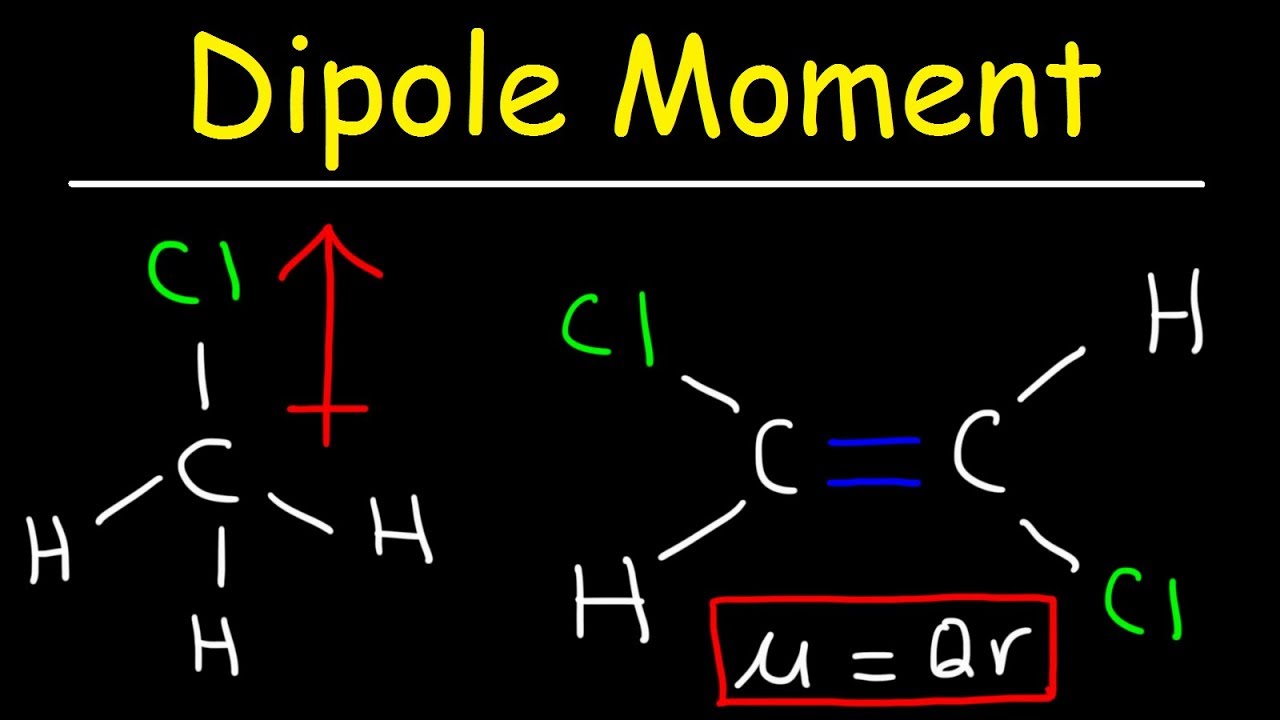
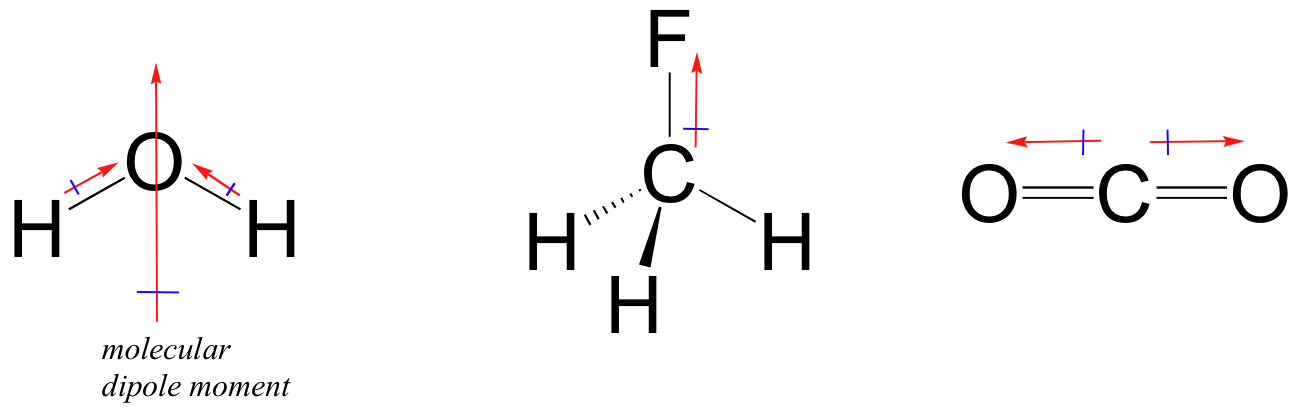
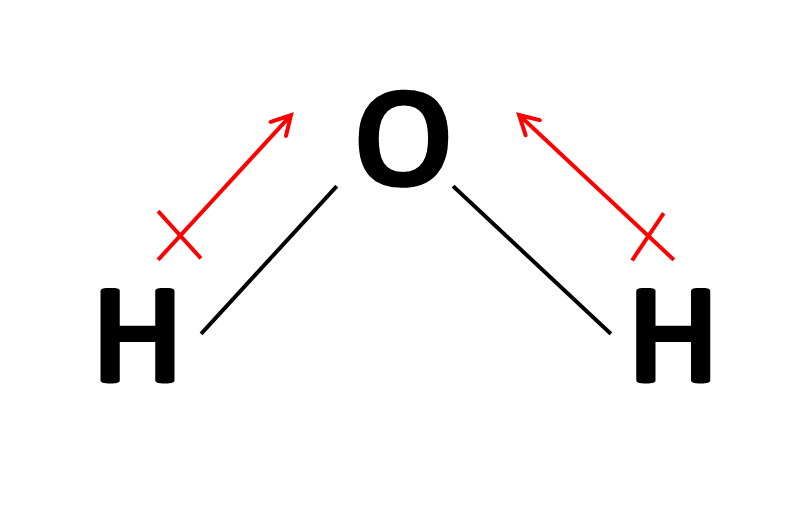
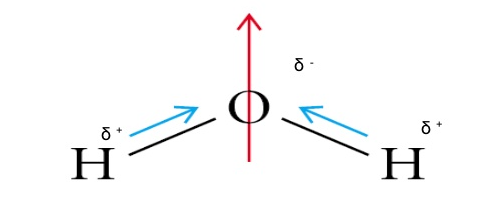
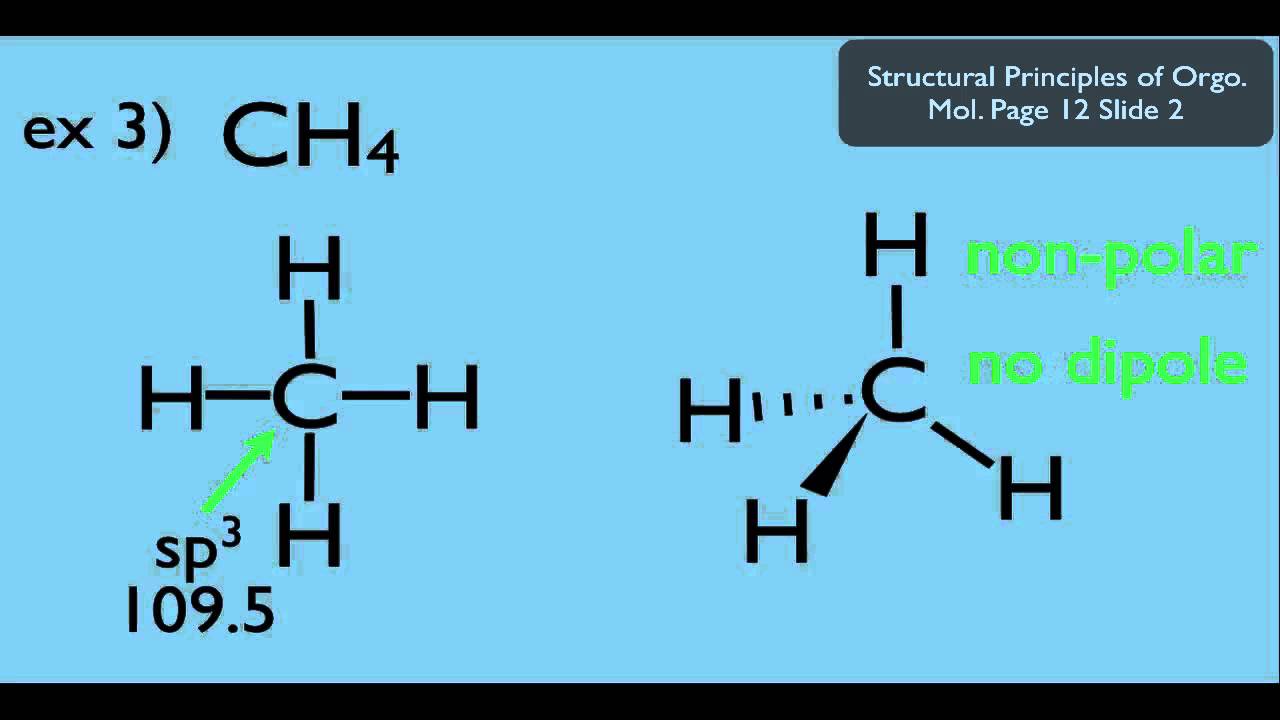
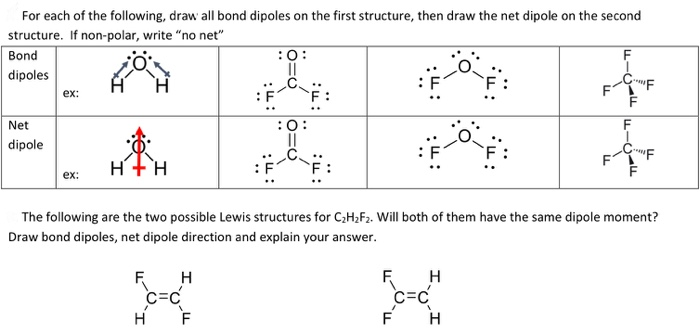
How to draw a dipole moment. The delta negative pole is the atom that is higher in electronegativity. Step 2) draw dipoles for each bond. Here are some quick steps to determine the dipole moment of the molecule:step 1) obtain the lewis dot structure for the molecule.
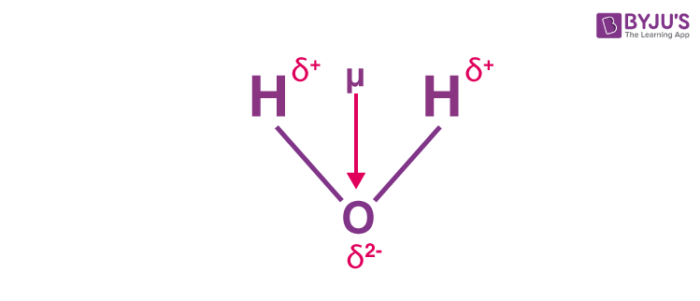
The mostly used unit of dipole moment is debye (d). Looking at the electronegativity and shape of the \(\mathrm{h}_{2} \mathrm{o}\) molecule tells you how the. Thus, the dipole moment, μ = q × d {\rm{\mu }} = q \times d μ = q × d;
Dipole moment in a lewis structure. It explains how to indic. This chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond.
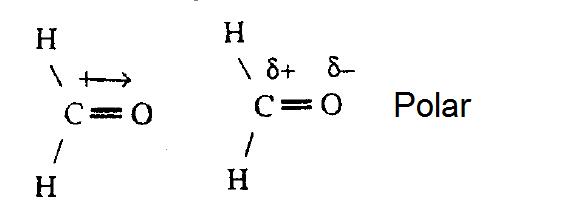
We draw dipole moments by using an arrow pointing towards the most electronegative atom that has a line running through it on the other end. It also explains how to calculate the percent. Q i is the magnitude of the i.
The symbols have their significant meanings. How to draw overall dipole moment november 25, 2021 a dipole moment is caused by the presence of one or more polar bonds ie bonds between two different elements. This organic chemistry video tutorial provides a basic introduction into dipole moment and molecular polarity.
The delta positive pole is the atom that is lower in electronegativity. Where, μ is the dipole moment vector.